Metabolic



Basics and background
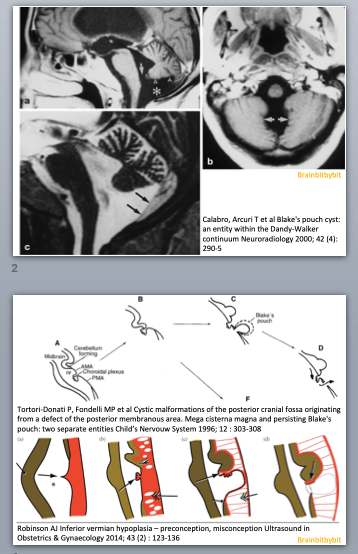
Ventricles, subarachnoid space and meninges
As a medical student the first thing about the brain I knew by heart was the ventricular system. It was so easy to remember: *Cerebrospinal fluid (CSF) gets produced by the choroid plexus in the lateral ventricles -> flows via the foramen of Monro to the 3rd ventricle -> from the 3rd ventricle via the aqueduct to the 4rd ventricle and then via L-Lateral foramina of Luschka and a M-Median foramen of Magendie to the subarachnoid space. *Granulations of Pacchioni in the subarachnoid space resorb CSF.
Problems were like plumbing, caused by blockage somewhere in the system.
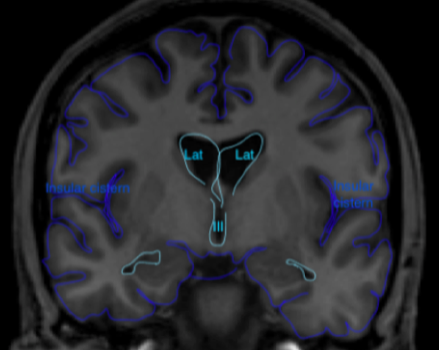
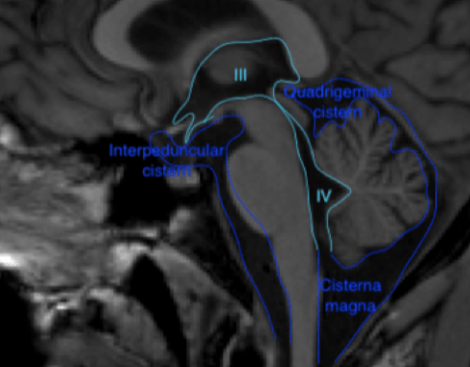
Figure 1 Coronal (above) and sagittal (below) T1 weighted images showing the lateral ventricles (lat), third (III) and fourth ventricle (IV) and several subarachnoid cisterns. The temporal horn of the lateral ventricle is anatomically close to the subarachnoid space, more specific the choroid fissure.
The brain and spinal cord are surrounded by 3 layers of connective tissue: (1) the pia, lying direct against the parenchyma (2) the arachnoid, which is an avascular layer bridging the cortical sulci and bridging the distance to the skull and (3) the dura mater consisting of an outer periosteal layer and an inner meningeal layer. The arachnoid and the space between the arachnoid and pia houses CSF. The widenings in the subarachnoid space called cisterns seemed like topography: something to memorize without the need for deeper knowledge.
About a decade later, as a fellow neuroradiology I dug deeper into the CSF circulation and concluded that hydrodynamics are not like plumbing, but more like predicting rain. Although the forementioned bulk flow model works fine in the majority of cases, it is not veracious and insufficient to explain more complicated conditions like normal pressure hydrocephalus. The compliance of the capillary vessel wall in combination with pressure gradients determines the filtration and absorption of CSF. CSF is (partly) actively secreted and the CSF protein composition differs from filtrated blood plasma. CSF is not absorbed in granulationes, which infants lack, but in brain capillaries.
Perivascular spaces and glymphatics
The pia and subarachnoid space continue around the arteries penetrating and perfusing the brain. There are topographic differences: the lenticostriatal arteries have a double sheet of leptomeninges and a perivascular space; cortical arteries are only covered by pia mater cells and do not have a perivascular space.
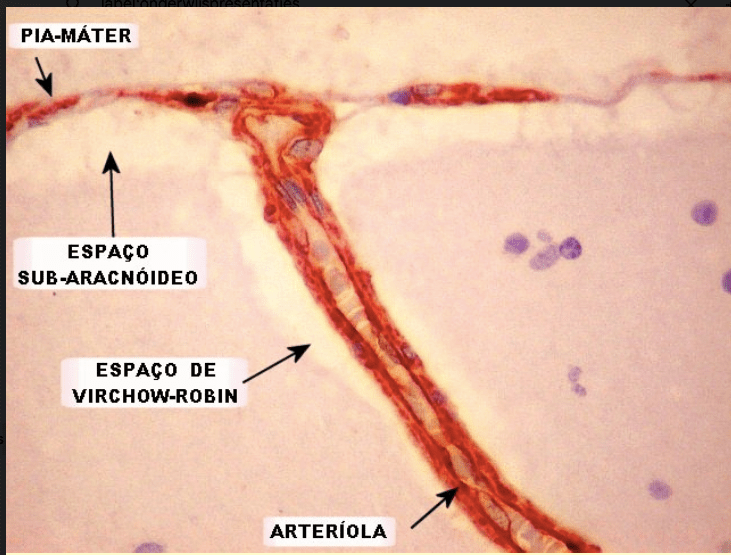
Figure 2. Histologic specimen demonstrating perivascular spaces a.k.a. Virchow-Robin spaces
This image is from the website http://anatpat.unicamp.br. I found it in 2008 or 2009 and used it for a presentation. I could not find this image on their (very informative) website in 2022, but might have overlooked it.
When going to smaller sized vessels from arteries and arterioles to capillaries, the pia mater turns into astrocyte endfeet. Astrocytes are a type of glial cells that have a common progenitor with oligodendrocytes, discussed in the section on “White matter”. Astrocytes play an important role in providing nutrients for the neurons, extracting glucose from the blood and delivering lactate to the neurons, and astrocytes have endfeet surrounding the endothelium of the brain’s capillaries. There is a gap, a space between the astrocyt endfeet where water and small solutes can pass paracellularly. Aquaporin-4 water channels (located in the astrocyte endfeet) transport water transcellularly and facilitate the flow from CSF into the brain interstitium. Cortical veins have paravenous (not perivenous!) channels as well.
Like lymphatics elsewhere in the body can absorb slightly larger molecules than the blood, the perivascular network in the brain functions as a macroscopic waste clearance system. Because of this analogy in 2012 the term “glymphatic system” for the brain’s perivascular network was introduced.
In the rest of the body, capillary walls consist of one layer of endothelial cells. In the brain the perivascular spaces and astrocytes provide an extra step from the capillary to the interstitium.
From a clinical point of view the two step barrier is not only important to understand neuro-inflammation, but also neurodegenerative diseases and cytotoxic edema in the pathogenesis of stroke. The glymphatic system received attention in mainstream media because one of the substances cleared by it is amyloid protein that plays a role in the pathogenesis of Alzheimer’s disease.